Cryogenic Safety: Basic Principle of Cryogenics
15 January 2026
Basic Principles of Cryogenics
Cryogenic systems handle gases liquefied at extremely low temperatures. Proper understanding of their physical properties, hazards, and operational characteristics is critical to ensure safe handling, installation, and operation. This section provides foundational knowledge for all personnel working with cryogenic fluids.
Overview of Cryogenic Fluids
Cryogenic liquids are gases cooled to very low temperatures, typically below -150°C (123 K), and stored as liquids. Their extreme cold and rapid expansion on vaporization present unique hazards.
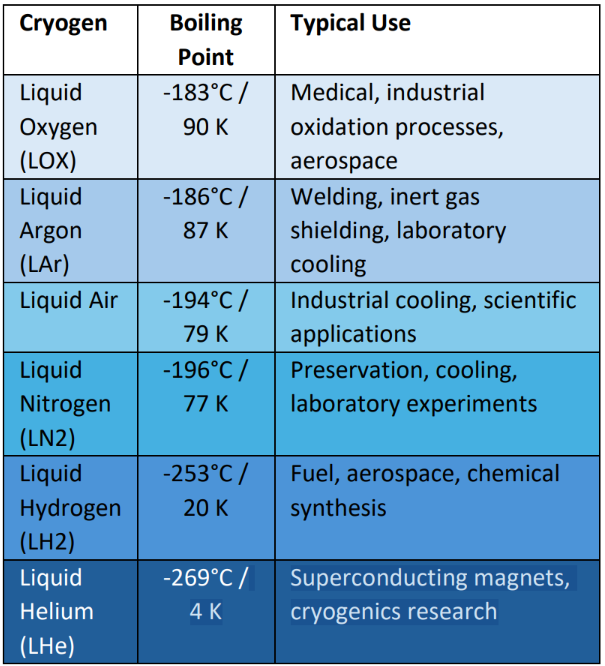
Physical Properties and Extreme Cold Behavior
Cryogens’ extreme low temperatures create hazards for personnel, materials, and equipment. Understanding these physical properties is crucial for safe handling.
| Property | Property | Safety Implications |
|---|---|---|
| Boiling Point | Temperature at which liquid vaporizes at 1 atm | Direct contact causes cold burns and frostbite |
| Liquid Density | Mass per unit volume | Impacts handling, storage, and transfer |
| Gas Density | Mass per unit volume at ambient conditions | Important for ventilation and oxygen depletion |
| Thermal Conductivity | Heat transfer rate | Rapid freezing of surfaces; cold burns |
| Specific Heat | Energy required to change temperature | Influences cooling and thermal shock |
| Material Interaction | Metals/plastics become brittle at low temperature | Risk of structural failure if incompatible materials are used |
Expansion and Vaporization Characteristics
Cryogenic liquids expand rapidly when vaporized. Understanding their expansion ratios and vaporization behavior is essential for designing safe systems and preventing hazards.
| Cryogen | Boiling Point | Liquid Density (kg/m3) |
Gas Density @ 1 atm, 25 °C (kg/m3) |
Liquid-to-Gas Volume Expansion Ratio | Hazard Notes |
|---|---|---|---|---|---|
| Liquid Oxygen (LOx) | -183 °C / 90 K | 807 | 1.1650 | ≈ 696 | Oxygen displacement; asphyxiation |
| Liquid Argon (LAr) | -186 °C / 87 K | 1141 | 1.4290 | ≈ 860 | Strong oxidizer; fire / explosion risk |
| Liquid Air | -194 °C / 79 K | 1400 | 1.7830 | ≈ 785 | Inert; oxygen depletion risk |
| Liquid Nitrogen (LN2) | -196 °C / 77 K | 870 | 1.2920 | ≈ 675 | Oxygen depletion; cold burn |
| Liquid Hydrogen (LH2) | -253 °C / 20 K | 71 | 0.0899 | ≈ 848 | Highly flammable; explosion hazard |
| Liquid Helium (LHe) | -269 °C / 4 K | 125 | 0.1786 | ≈ 848 | Asphyxiation risk; extreme cold |
Back
